Treatment for Severe Emphysema
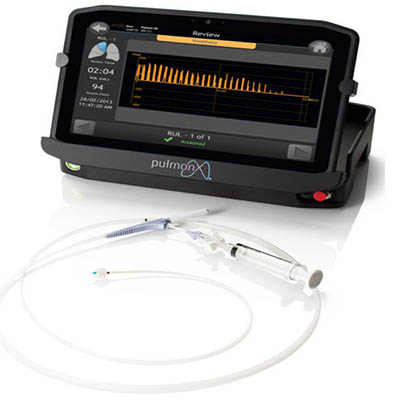
The vision of Pulmonx is to be a global leader in bronchoscopic treatment for emphysema. The Zephyr® Valve System has clinically-proven results, reliability, patient selection tools, and ease of use.
Zephyr Valve
How it Works
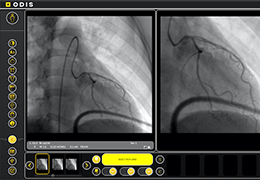
Minimally Invasive Bronchoscopic Procedure
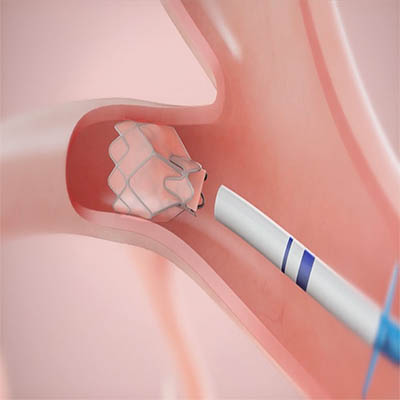
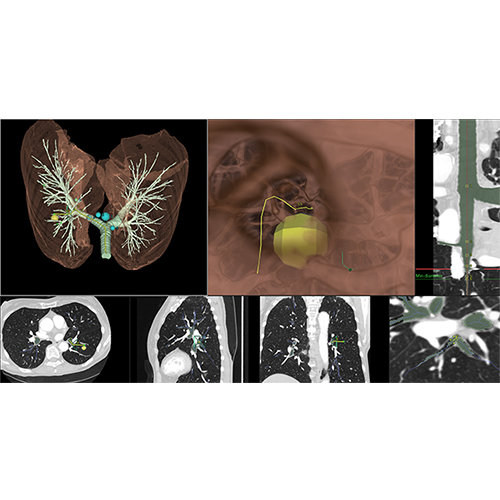
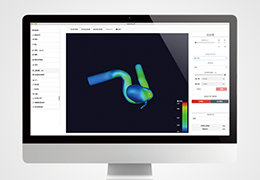
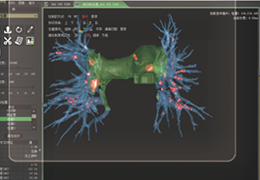
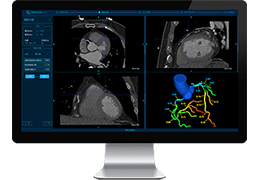
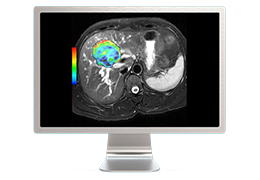
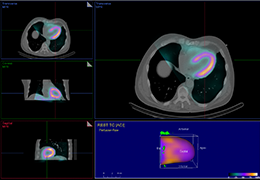
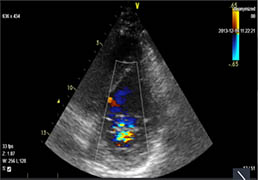
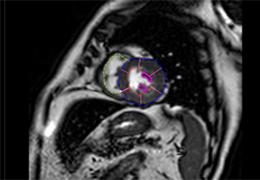
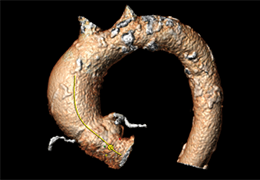
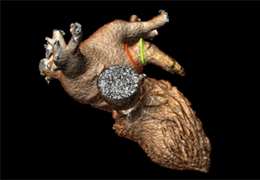
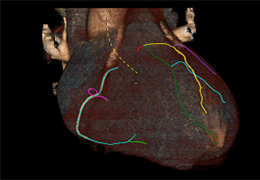
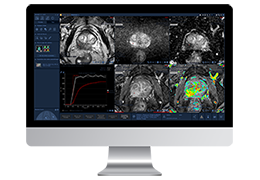
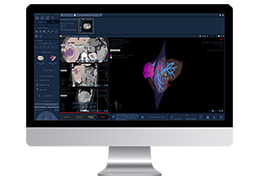
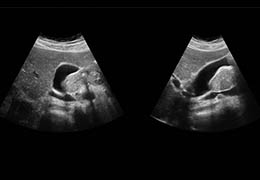
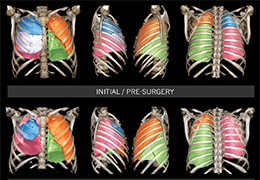
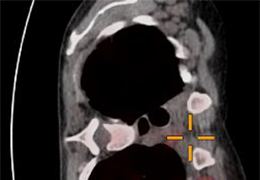
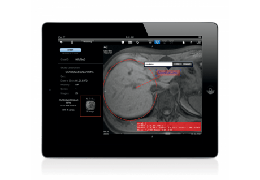
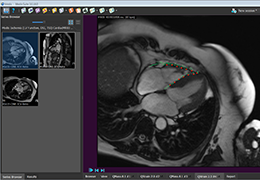
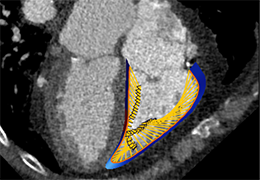
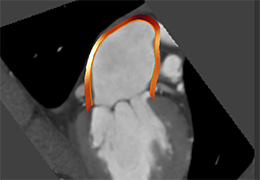
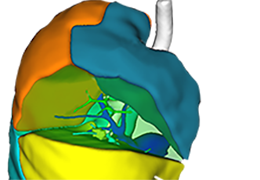
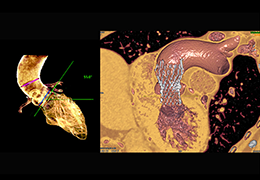
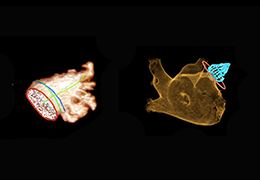
The Zephyr Endobronchial Valve was granted a “breakthrough medical status”20 by the FDA and is indicated for bronchoscopic treatment of patients with hyperinflation associated with severe emphysema in regions of the lung that have little to no collateral ventilation (CV). The Zephyr Valve is an implantable device used to occlude all airways feeding the hyperinflated lobe of a lung that is most diseased with emphysema.
Trapped air escapes through the Zephyr Valves until the lobe volume is reduced. The remaining lobes are then able to expand more fully and work more efficiently, reducing pressure on the diaphragm and improving overall lung function.
30 to 60-minute procedure
Typically, 3 to 5 valves placed to completely occlude the lobe
Can be removed or replaced if needed
3-night stay in the hospital
Complications of the Zephyr Endobronchial Valve treatment can include, but are not limited to, pneumothorax, worsening of COPD symptoms, hemoptysis, pneumonia,
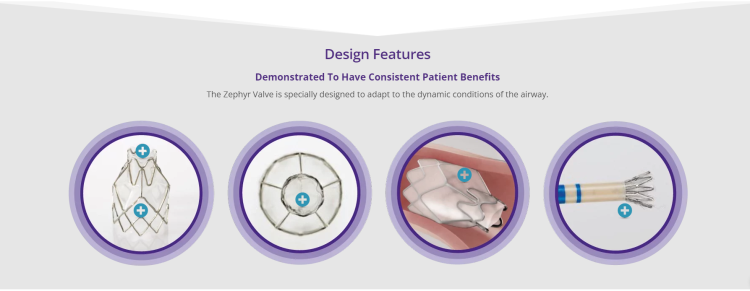
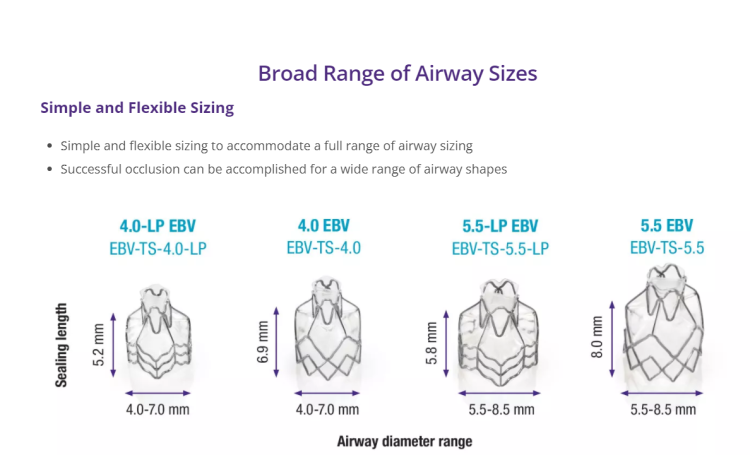
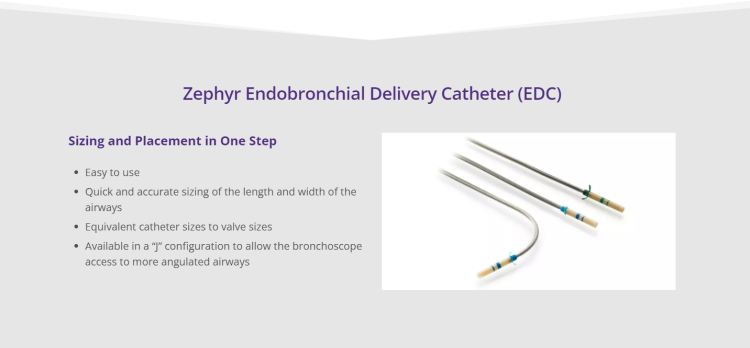
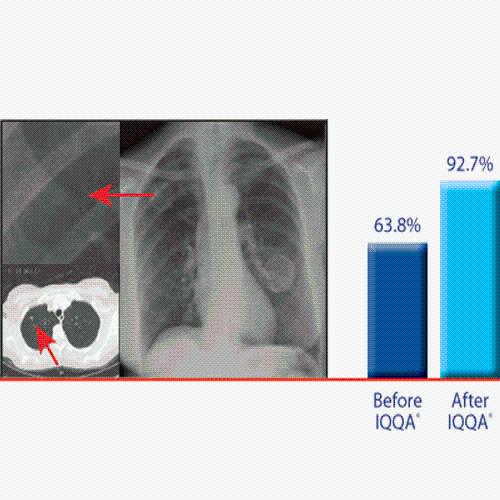
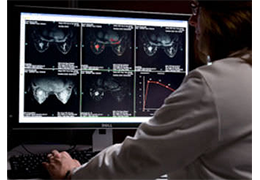
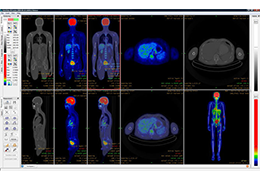
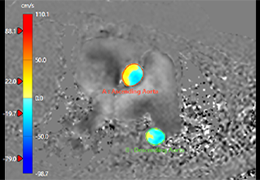
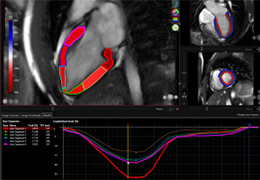
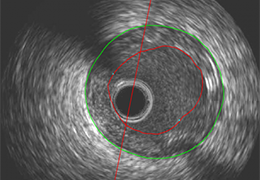
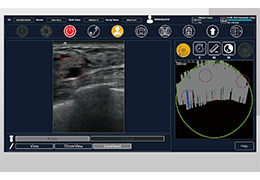
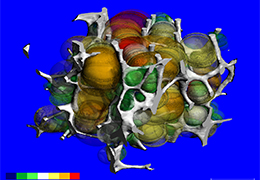
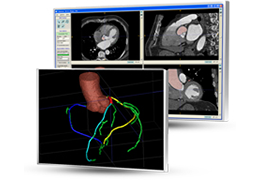
Technology for Precise Patient Selection
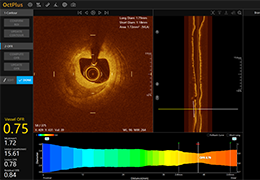
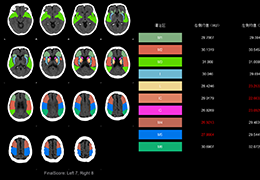
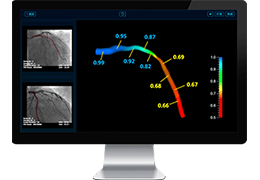
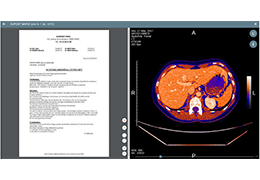
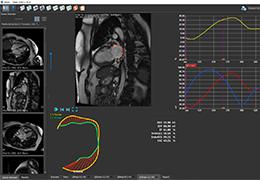
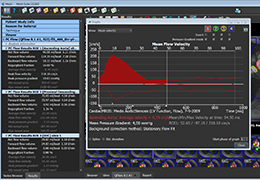
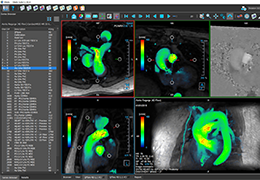
Appropriate patient selection is critical to the success of Zephyr Valve treatment. Clinical trial data have helped define the successful patient profile.17
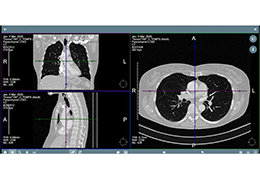
Prior to treatment, physicians perform a clinical workup, including lung function and hyperinflation testing. In addition, Pulmonx offers assessment tools that precisely identify patients who are most likely to benefit from Zephyr Valve treatment.
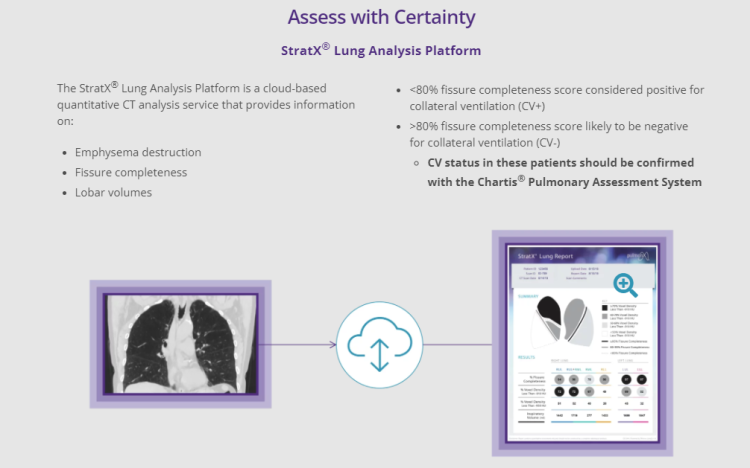
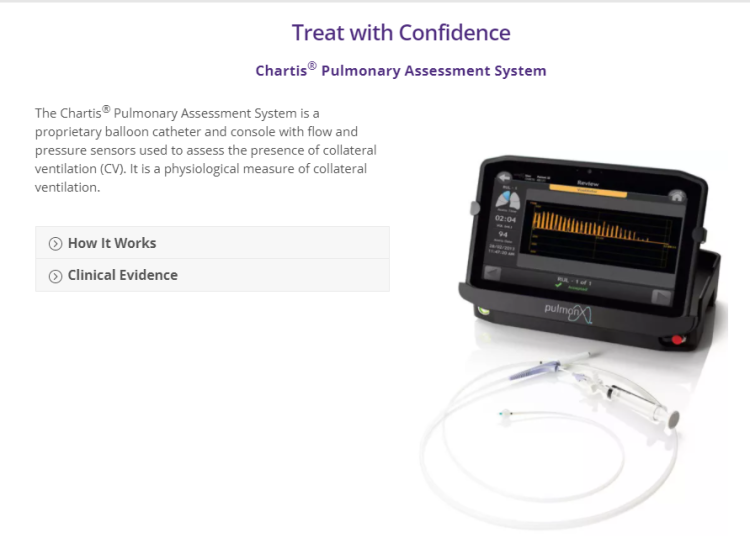
 了解更多
了解更多
 了解更多
了解更多